First responders and people who participate in outdoor activities, such as mountaineering and hiking, should check if they have a SAM XT Extremity Tourniquet and return it to their distributor for a new one.
A problem in the sewing operations has been identified which could cause the seam holding the buckle to the belt to fail when used on a patient to stop blood flow. Although not widely used in the UK, tourniquets are used for critical, emergency situations, and so it’s important people are aware if they own an affected product.
Only a small number of lots are being recalled. This product has been sold worldwide since March 2017. No other types of tourniquets are affected.
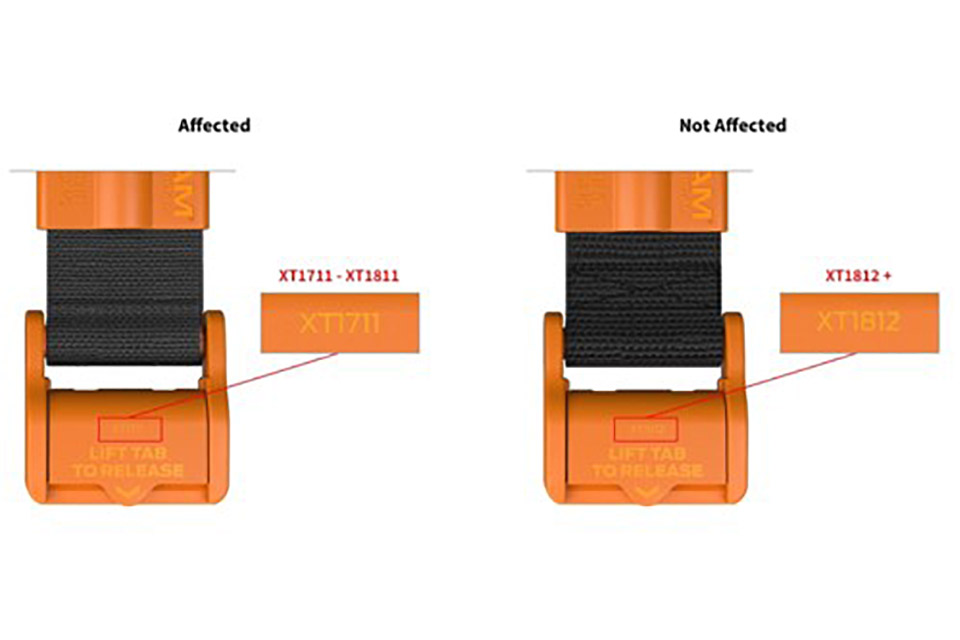
The SAM XT devices affected by the recall can be identified by:
- The absence of a “Box X Stitch” on the Instructions For Use (IFU)
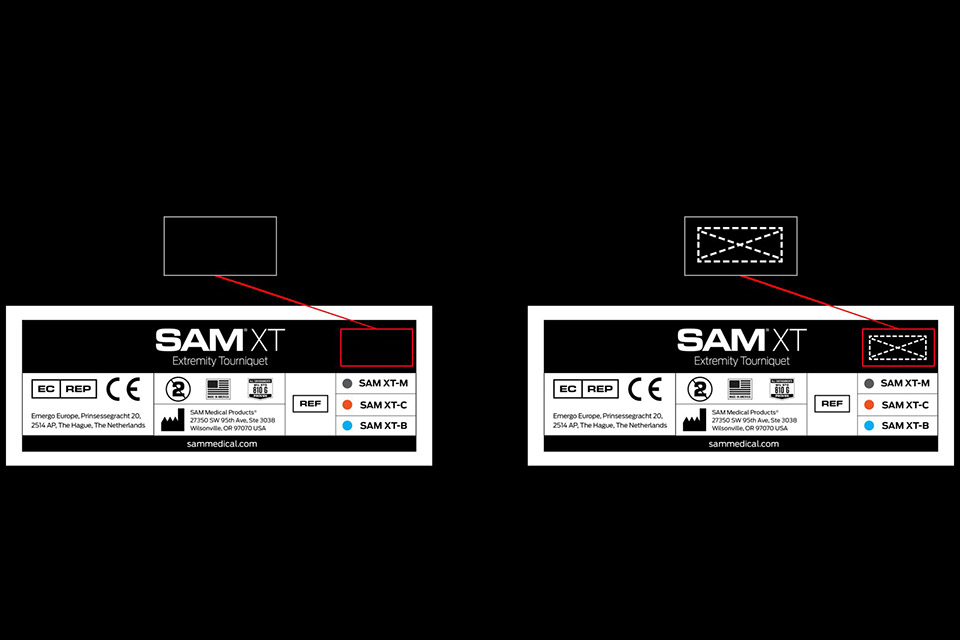
- The absence of a “Box X Stitch” on the device

- Any Lot Number with XT1812 or higher
John Wilkinson, the Medicines and Healthcare products Regulatory Agency’s (MHRA) Director of Medical Devices, said:
Check if you have the listed tourniquet and if you do so, please separate the device from your pack and take them back to where you bought them from.
Our highest priority is making sure medical devices are safe. This is why we are supporting the company to carry out this recall and why we want people to check their inventory.
As with any medical device, we strongly encourage anyone to report any suspected manufacturing faults to us via our Yellow Card Scheme.
Notes to Editor
- MHRA is responsible for regulating all medicines and medical devices in the UK. All our work is underpinned by robust and fact-based judgments to ensure that the benefits justify any risks. MHRA is a centre of the Medicines and Healthcare products Regulatory Agency which also includes the National Institute for Biological Standards and Control (NIBSC) and the Clinical Practice Research Datalink (CPRD). The Agency is an executive agency of the Department of Health. www.mhra.gov.uk
- Link to Yellow Card Scheme
- Medical Device Alert
- SAM Medical company website recall
Media enquiries
News centre
MHRA
10 South Colonnade
London
E14 4PU
Email
newscentre@mhra.gov.uk
During office hours:
020 3080 7651 (08:30 – 17:00)
Out of office hours:
07770 446 189 (17:00 – 08:30)
Office hours are Monday to Friday, 8:30am to 5pm. For real-time updates including the latest press releases and news statements, see our Twitter channel at https://www.twitter.com/mhrapress
Follow this news feed: Public Health





